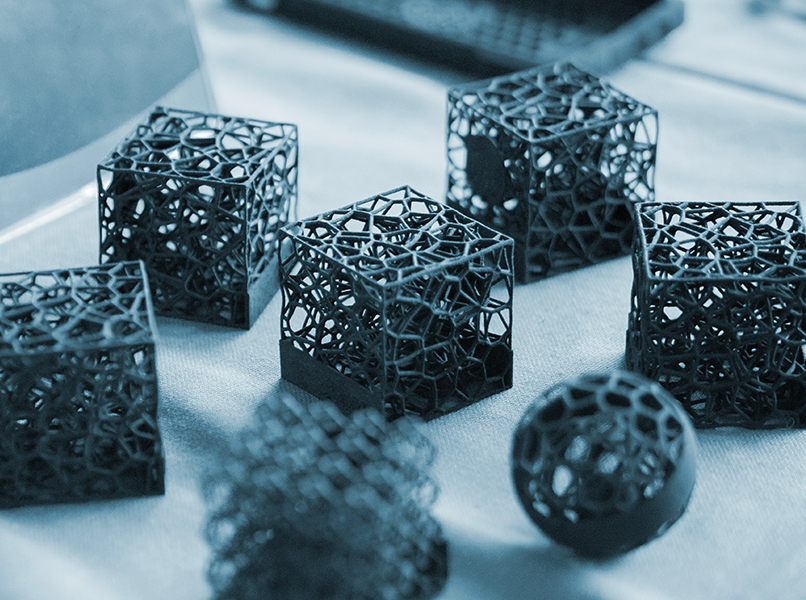
Leverage the Latest in 3D Printing to Speed Time to Market
This session takes a deep dive into the printing technologies that can streamline production cycles and bring innovative products to market faster than ever before.

This session takes a deep dive into the printing technologies that can streamline production cycles and bring innovative products to market faster than ever before.

Explore ways to work with a contract manufacturer to identify, analyze and implement sustainable spending reduction strategies and improved inventory management.

Additive manufacturing suppliers are building devices with bioresorbable polymers, tantalum, bioceramics and advanced metal alloys that improve biomechanics, enhance fixation and help the body heal.

Join this engaging and informative session to understand various techniques for wear testing, hybrid manufacturing and tumble finishing and polishing. During this rapid-fire session, presenters will share case studies that highlight best practices in additive and subtractive manufacturing.

This presentation discusses chlorine dioxide gas sterilization, the technology and science behind it, and its benefits over other sterilization methods. Through a case study on a knee system, you'll learn about the implementation, validation and effectiveness of the chlorine dioxide process.
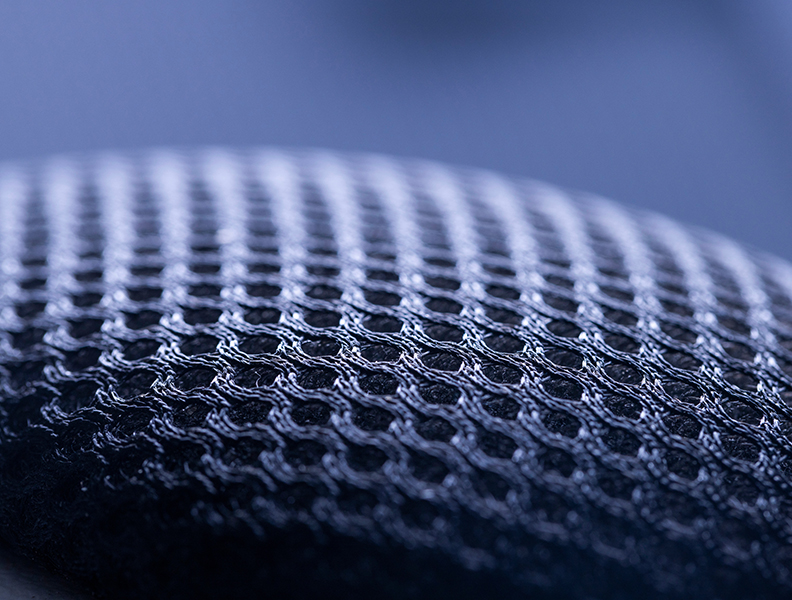
The marketplace is looking for key features of nanotechnology, including antimicrobial and anti-inflammatory properties, vascular recruitment and promoting osseointegration.
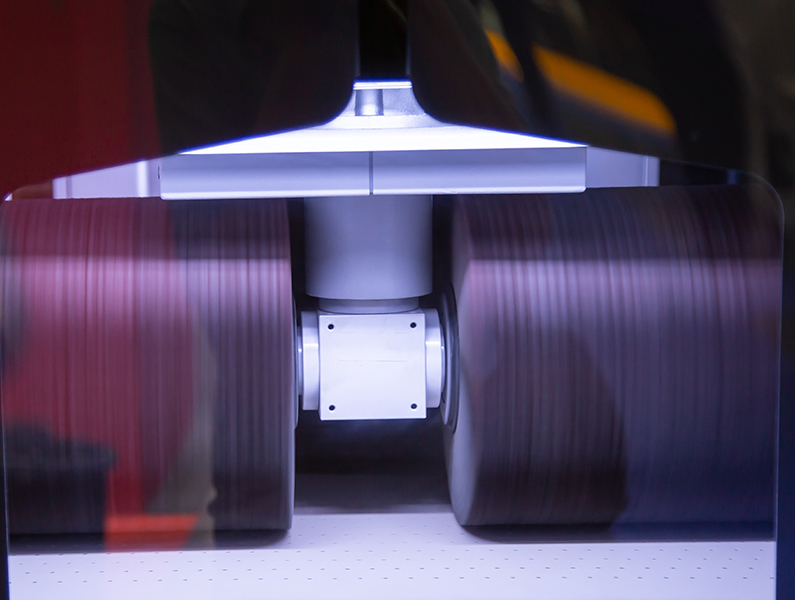
Ultrasonic deburring is a groundbreaking new process that eliminates the disadvantages of other deburring methods while delivering optimum results without material removal. This presentation demonstrates ultrasonic deburring and shares how it's used on metals and plastics.